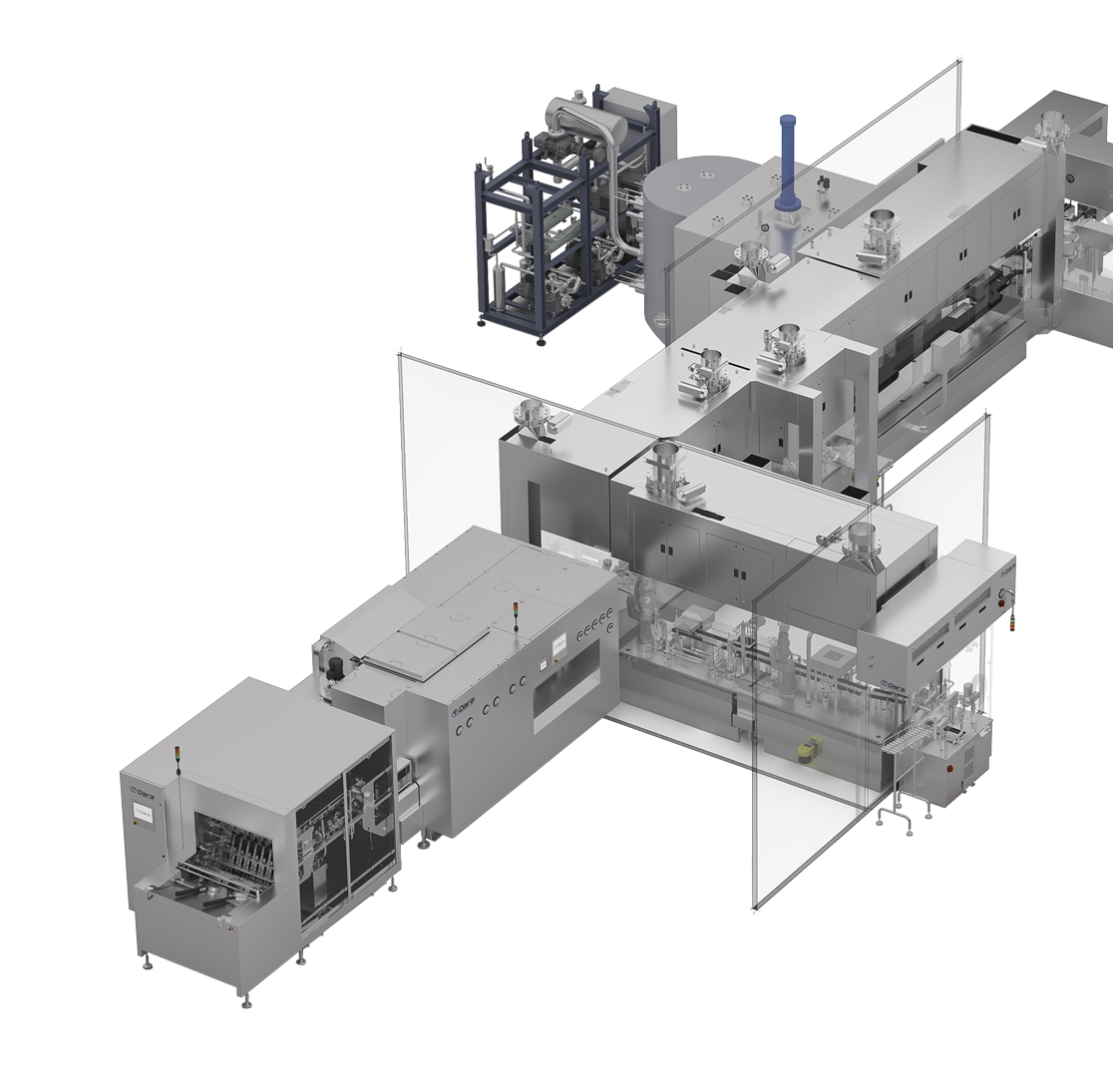
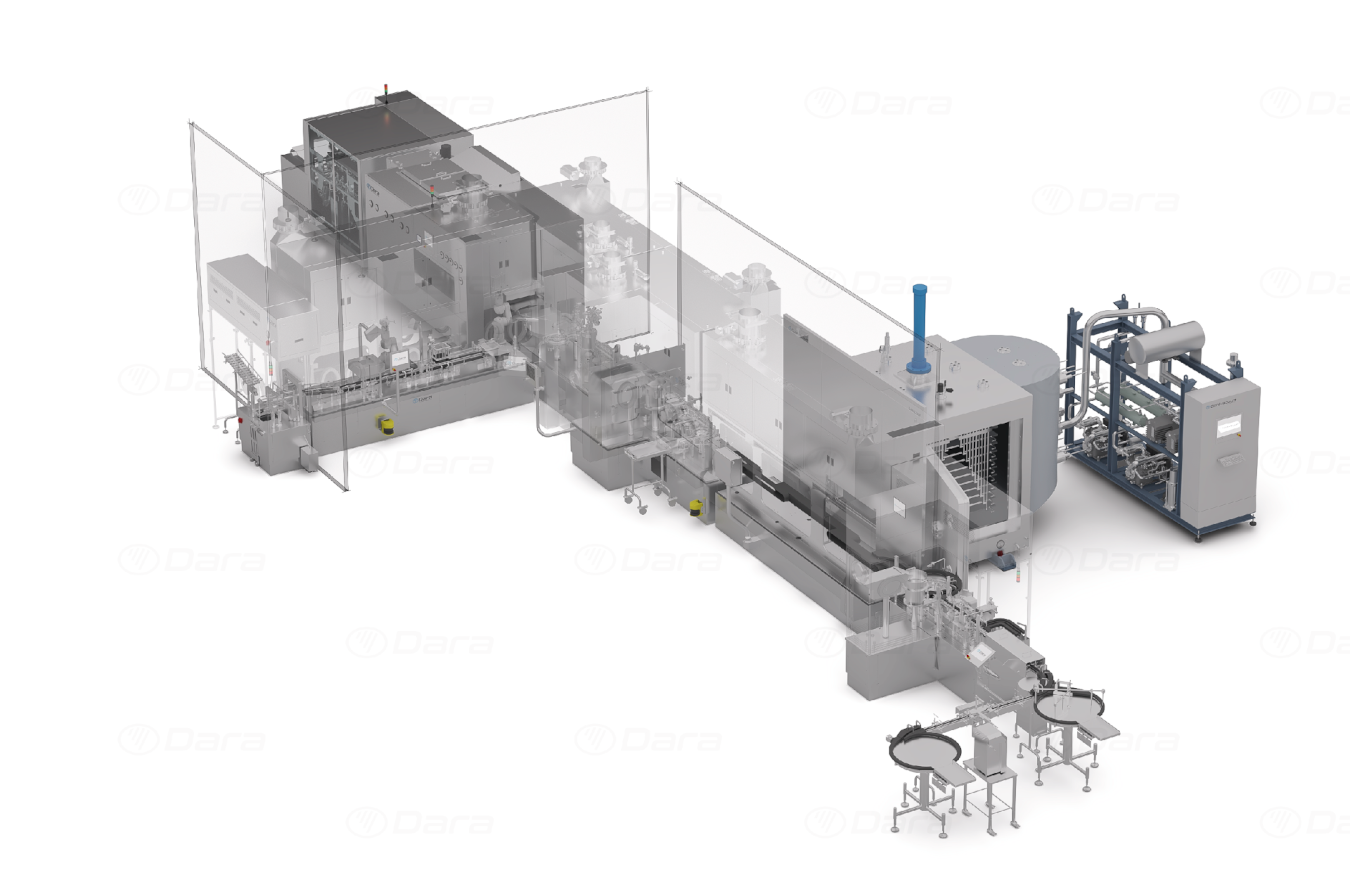
Multiformat-line-for-aseptic-packaging
Flexibility: The Key to Success
The continuous evolution and development of new inactivated, attenuated, or viral vector-based vaccines, as well as their dispensing methods, make adaptation to a versatile aseptic packaging system essential. Dara Pharma has evolved in this direction, integrating complete lines, Turn-Key Solutions, which make the vaccine packaging process more profitable.

Multiformat
Great versatility with different types of packaging —vials, cartridges, and syringes—, and quick format changeover.

Bulk & RTU
For formats supplied in bulk and RTU (Ready-to-use).

Multiproduct
Dosing of liquid and powder products, as well as integration with a freeze drying process.
Multiformat line for aseptic packaging
- Washing and sterilization of bulk vials
- Debagging, delidding and denesting in RTU formats
- Filling and closing of vials, syringes, and cartridges for liquid and powder injectables
- Freeze drying and closing of vials for freeze dried injectables



Bulk vial infeed
The sequence of vial processing in bulk starts with the loading of vials into the washer.
More information
Washing
Dara Pharma's linear and rotary washing machines have been designed for maximum efficiency, low consumption, and high flexibility to process the maximum number of formats.
More information
Depyrogenation
Dara Pharma's modular sterilization and depyrogenation equipment has been designed to process glass containers efficiently and continuously. In addition to being easily adaptable to customer requirements, these solutions provide lower energy consumption in production.
More information
Debagging for RTU formats
The processing sequence of RTU vials, syringes and cartridges starts with the debagging module, which automatically and efficiently removes the bag from the nest or tray.
More information
Delidding
The processing of RTU formats continues with the delidding, in which the Tyvek lid and the inner liner that protects the contents of the nest or tray are removed.
More information
Denesting
The compact denesting equipment allows fast and accurate unloading of vials, syringes, and cartridges for subsequent individual processing.
More information
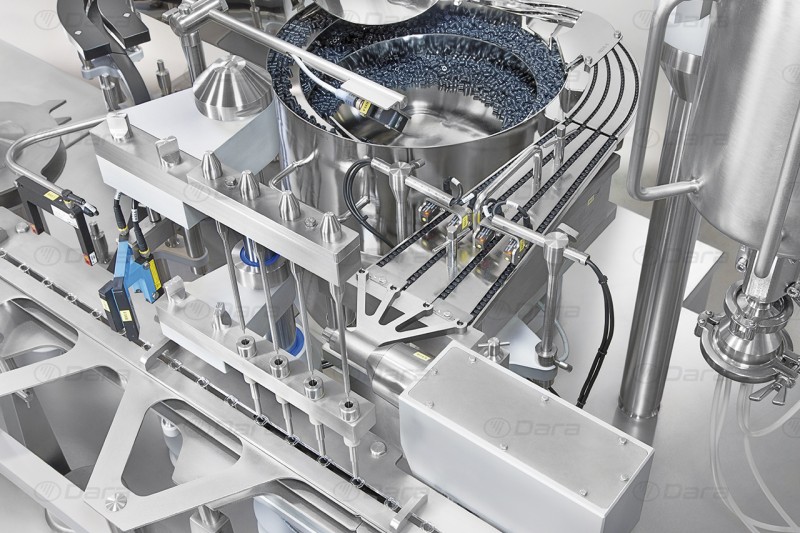
Filling and closing
Dara Pharma's aseptic filling solutions stand out for their versatility and precision in the dosing of vials, syringes and cartridges for liquid and powder products. The closing machines control and measure multiple parameters during this process to guarantee the complete tightness of the product, such as crimping pressure, closing height, or any visual control.
More information
Loading and unloading systems
Automatic vial loading and unloading systems have been designed to connect the filling line with the freeze dryer. The implementation of this equipment aims not only to increase the efficiency of the line, but also to reduce potential cross-contamination in this process.
More information
Freeze drying
Lyophilization is a drying process that removes solvent by sublimation and not boiling like other drying processes. Sublimation is a product changing from a solid to a gas without passing through an intermediate liquid phase. As a result, the product with much more powerful active ingredients and nearly immediate reconstitution time is obtained.
More information
Closing
Once vials have been freeze dried, the high-speed linear closing machine guarantees that any product complies 100% with the established requirements using different types of aluminum closures.
More information
External washing
The external washing machine is incorporated at the end of the filling line to achieve an external cleaning of the containers to remove any impurities.
More informationBulk processing
The line has a washing machine and a sterilization and depyrogenation tunnel for bulk supply.
Cleaning
WM / RWM
Maximum efficiency and high flexibility to process as many formats as possible: vials, bottles, or cartridges.
Sterilization & Depyrogenation
DT
Maximum flexibility and adaptability, low consumption, and easy maintenance thanks to its modular design.
Linear washing unit
for vials
WM
- Multiformat machines
- Quick format changeover
- Output up to 36,000 uph
- Pre-washing cycle with ultrasound bath.
- Steam sterilization of pipes.
- Drying-in-place of pipes.
- Siliconizing cycle.
- Automatic rejection of defective containers and extraction for sampling.
- SCADA software to process data acquisition in accordance with FDA 21CFR Part 11.
- IQ / OQ Validation package.
Rotary washing machine
for vials and cartridges
RWM
- Multiformat machines
- Quick format changeover
- Output up to 24,000 uph
- Pre-washing cycle with ultrasound bath.
- Steam sterilization of pipes.
- Drying-in-place of pipes.
- Siliconizing cycle.
- Automatic rejection of defective containers and extraction for sampling.
- SCADA software to process data acquisition in accordance with FDA 21CFR Part 11.
- IQ / OQ Validation package.
Sterilization and depyrogenation tunnel
DT
- Continuous process
- Automatic format control and adjustment
- Output up to 610 kg/h
- Differential pressure control.
- Clean room sealed door.
- Automatic tunnel discharge.
- Cooling chamber sterilization.
- Automatic adjustment of chambers doors opening.
- Air speed monitoring.
- SCADA software to process data acquisition in accordance with FDA 21CFR Part 11.
- IQ / OQ validation package.
RTU processing (Ready-to-use)
Debagging, delidding and denesting are available for supply in nest and tray.
Debbaging
DB
Deliding
DL
Denesting
DN
Automatic debagging
DB/A
- Automatic opening of the bag
- Automatic transfer of the tub to a downstream machine through an automatic sliding door
- Output up to 225 tubs/h
- SCADA software to process data acquisition in accordance with FDA 21CFR Part 11.
- GAMP complying software.
- Laminar air flow / RABS.
- IQ / OQ Validation package.
Automatic delidding
DL/A
- Tub pre-heating to avoid particle generation during Tyvek lid removal
- Inner liner removal through vacuum
- Output up to 225 tubs/h
- SCADA software to process data acquisition in accordance with FDA 21CFR Part 11.
- GAMP complying software.
- Laminar air flow / RABS / Isolator.
- IQ / OQ Validation package.
Automatic denester
DN/A
- Compact machine
- Quick format changeover
- Output up to 24,000 uph
- SCADA software to process data acquisition in accordance with FDA 21CFR Part 11.
- “No glass-to-glass contact” denester.
- Monitoring and particle counting.
- Laminar air flow / RABS / Isolator.
- IQ / OQ Validation package.
Filling
Dara Pharma's filling solutions have revolutionized the aseptic packaging machinery sector thanks to their versatility and flexibility. With our solutions, it is possible to process liquid, semi-solid and powder products in the same equipment.
|
Peristaltic
dosing |
Volumetric
dosing |
Time-pressure
dosing |
|
Liquid |
|||
| Pressure-vacuum dosing |
Powder |
Closing
Closing is the operation that guarantees the seal of the container. Dara Pharma's closing machines control and measure the parameters required in this process, such as torque, crimp pressure, closing height, and any visual control – guaranteeing that the products are closed in a satisfactory way.
Multiformat filling and closing machine
VSC
- Filling from 0.1 - 100 ml
- 100% IPC
- Output up to 24,000 uph
- Washing unit and sterilization tunnel for vials and cartridges supplied in bulk.
- Denesting unit for RTU (Ready-to-use) formats.
- Weight dosing control for 100% of processed elements.
- Gas flushing before, during or after the filling process.
- Dosing system for CIP / SIP conditions.
- Automatic rejection of defective containers.
- SCADA software to process data acquisition in accordance with FDA 21CFR Part 11.
- Monitoring and particle counting.
- Laminar air flow / RABS / Isolator.
- IQ / OQ Validation package.
Freeze drying
Lyophilization is a drying process that removes solvent by sublimation and not boiling like other drying processes. Sublimation is a product changing from a solid to a gas without passing through an intermediate liquid phase. As a result, the product with much more powerful active ingredients and nearly immediate reconstitution time is obtained.
Extends
shelf life
Extends the shelf life of the product.
Easy
reconstitution
The rehydration process is instantaneous.
Maximization
of properties
Maintains the qualitative properties of the product.
Freeze drying
Loading system + Freeze dryer
Automatic loading and unloading systems
for vials
VLS (Vial Loading System)
- Output up to 24,000 uph
NLS (Nest Loading System)
- Output up to 115 nest/h
GMP Freeze Dryers

LyoCompact
- From 2 to 6 m² of shelf area
- Condenser capacity up to 80 kg
- Output for 2R up to 28,000 vials
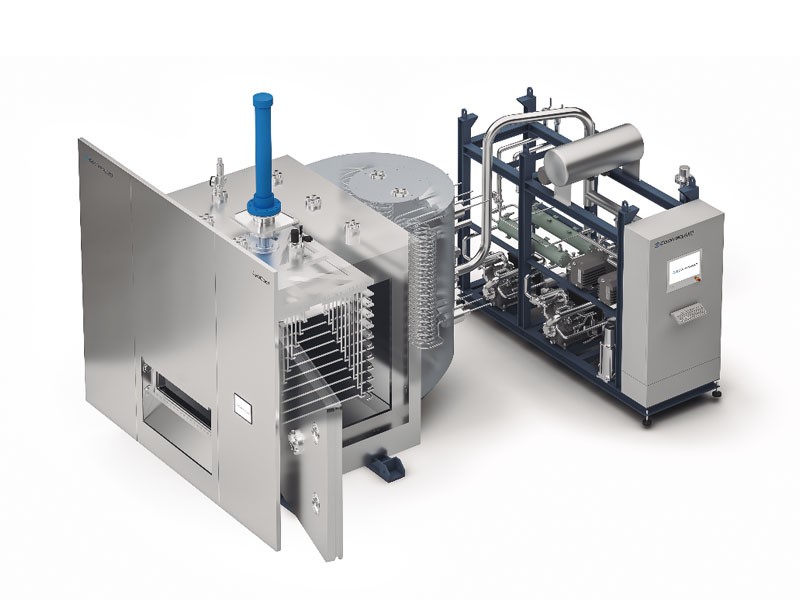
LyoCool
- From 7 to 15 m² of shelf area
- Condenser capacity up to 250 kg
- Output for 2R up to 65,000 vials

LyoPro
- From 16 to 45 m² of shelf area
- Condenser capacity up to 800 kg
- Output for 2R up to 200,000 vials
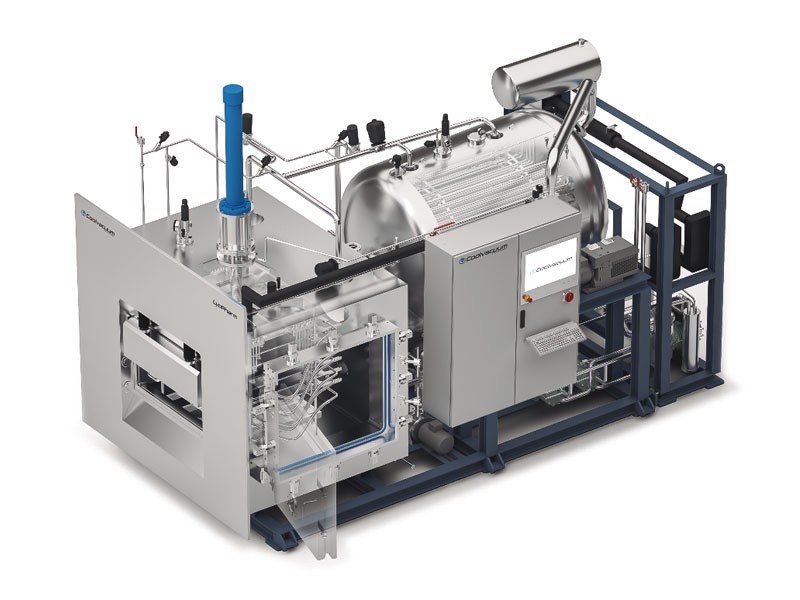
LyoPharm
- From 2 to 45 m² of shelf area
- Condenser capacity up to 800 kg
- Output for 2R up to 200,000 vials

External closing and washing
of freeze dried vials
Closing
HSL-CAP
External washing
EWM
High speed vial closing machines
HSL-CAP/8
- Compact machine
- Minimal particle generation during crimping
- Output up to 36,000 uph
- Washing unit and sterilization tunnel for vials supplied in bulk.
- Automatic rejection of defective vials.
- SCADA software to process data acquisition in accordance with FDA 21CFR Part 11.
- Laminar air flow / RABS / Isolator.
- IQ / OQ Validation package.

External washing unit
EWM
- Output up to 24,000 uph
- For 1 to 500 ml vials
Advantages of full line integration
1
Mechanical integration between machines
Design, manufacture, and adjustment of the transfer systems for the correct integration of all the machines of the line.
2
Software architecture and single control
A control system is configured for the entire line, so that all the machines operate under the same software and data acquisition system.
3
Complete service and maintenance
The complete maintenance plan for the entire line ensures that all machines will operate optimally and efficiently in the long term.
Contact
Dara Pharma
We have a team of specialists waiting to answer all your questions and inquiries. Feel free to contact us through this form and we will get in touch with you.